Toray Industries, Inc.
Tokyo, Japan, May 9, 2022 – Toray Industries, Inc., announced today that wholly owned subsidiary Toray Medical Co., Ltd., has secured insurance coverage for the SATAKE・HotBalloon™ ablation catheter to treat drug refractory recurrent symptomatic persistent atrial fibrillation in Japan.Toray is authorized domestically to manufacture and sell this product.
Atrial fibrillation is an irregular and often very rapid heart rhythm from abnormal electrical signal in the atria. There are four main types of such fibrillation; paroxysmal (terminates within 7 days), persistent (sustained beyond 7 days), long-standing persistent (sustained for at least 12 months) and permanent atrial fibrillation. Around 70,000 ablations for atrial fibrillation are performed in Japan each year (see notes 1 and 2). An estimated 20% of those procedures are for the persistent type (see note 3).
Toray secured marketing approval in 2015 for the SATAKE・HotBalloon™ ablation catheter to treat drug refractory recurrent symptomatic paroxysmal atrial fibrillation. There has been a growing need in recent years to also use this product for persistent atrial fibrillation. |
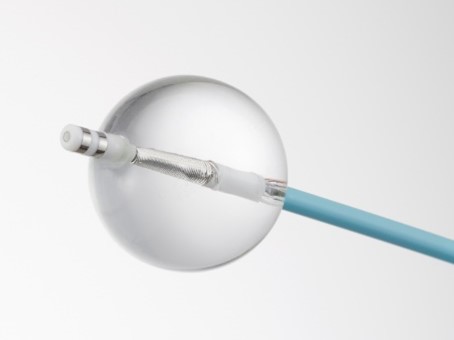 |
The company accordingly obtained marketing approval in October 2021 to add HotBalloon™ to indications for treating drug refractory recurrent symptomatic persistent atrial fibrillation, with insurance coverage beginning in May this year.
Toray expects this additional indication can be a new treatment option for persistent atrial fibrillation patients.
Toray and Toray Medical will keep contributing to atrial fibrillation treatment progress by developing and providing safe and reliable offerings that match market needs.
Notes
| 1. |
Ministry of Health, Labour and Welfare, 3rd through 5th Open Data K Surgery K595 Percutaneous catheter myocardial ablation. |
| 2. |
T. Yamane, M. Shoda, and A. Nogami, Expanding Indications for Balloon Ablation for Persistent Atrial Fibrillation, Electrocardiography, vol. 40, no. 2, pp. 67-68, 2020. |
| 3. |
Y. Murakawa, Nationwide Survey of Catheter Ablation for Atrial Fibrillation: The Japanese Catheter Ablation Registry of Atrial Fibrillation (J-CARAF), Circ J, vol. 78, pp. 1091-1096, 2014. |
Product Details
| Brand name: |
SATAKE・HotBalloon Catheter |
| Generic name: |
Catheter for cardiac ablation |
| Approval number: |
22700BZX00355000 |
| Category: |
Class IV specially controlled medical device |
| Reimbursement category: |
123 Percutaneous catheter for myocardial ablation (1) For thermal ablation ③ Balloon type |
| Description: |
A catheter developed in Japan for thermal balloon ablation. A high-frequency electric current heats liquid in the balloon on the catheter tip. The heat transfers to the treatment location, which is the left atrium myocardial tissue. The balloon is flexible and can vary in size, so it can enter the pulmonary vein, the prime atrial fibrillation source, ablating circumferentially. HotBalloon™ should contribute significantly to short-time treatment of atrial fibrillation that is safe and effective. |