- HOME
- Sustainability
- Contributing Solutions to Social Issues through Business Activities
- Life Innovation Business Expansion Project
CSR Activity Report (CSR Guideline Activity Reports) - Contributing Solutions to Social Issues through Business Activities
Life Innovation Business Expansion Project
Revenue of Life Innovation products (IFRS)
- ■Reporting scope
- Toray Group
- ■Target
- 300.0 billion yen(Fiscal 2022)
Fiscal 2022 Result
369.6
billion yen
In the field of health and medical care, the world has entered a period of historic change. The threats posed by the COVID-19 pandemic and the impact of climate change on healthcare have been added to the existing challenges of declining birthrates and aging populations in developed countries, climbing social security costs, and global healthcare disparities.
Toray Group’s life science business helps to support health and medical care, especially through polymer material research, which Toray Group has pursued since its establishment.
The Life Innovation Business Expansion Project started in fiscal 2014 with the launch of the Medium-Term Management Program, Project AP-G 2016. Life Innovation is a group-wide project aimed at improving health by making the most of Toray Group’s advanced materials, core and elemental technologies, and business platforms. The project focuses on businesses that can improve the quality of medical care, reduce the burden on medical staff, and support people’s health maintenance and longevity.
Furthermore, under the Medium-Term Management Program, Project AP-G 2022, which was initiated in fiscal 2020, Toray Group has been adding businesses related to personal safety products, including ones that help protect people from infectious diseases, extreme weather (heat waves, etc.), disasters, and accidents, while strengthening these businesses.
Product Definitions and Guidelines
Improving the quality of medical care and reducing burden on medical staff
- Products used in medical treatment, products used in medical testing and diagnosis, supplies/products used in medical institutions
Supporting a society where people everywhere can live long, healthy lives
- Maintaining wellness, health, and independent living, improving activities of daily living (ADLs) for the elderly and home-care recipients, reducing the burden on care givers (nursing staff and families), and addressing public health issues
Supporting personal safety
- Leveraging materials to protect people from disasters, extreme weather (heat waves, etc.), accidents, and infectious diseases
With the addition of personal safety products to the Life Innovation business area in fiscal 2020, net sales (“revenue” from fiscal 2020) for these businesses have steadily increased from 142.2 billion yen in fiscal 2014 to 369.6 billion yen in fiscal 2022, surpassing the target of 300 billion yen for that year. The level has reached 15% of Toray Group's consolidated revenue.
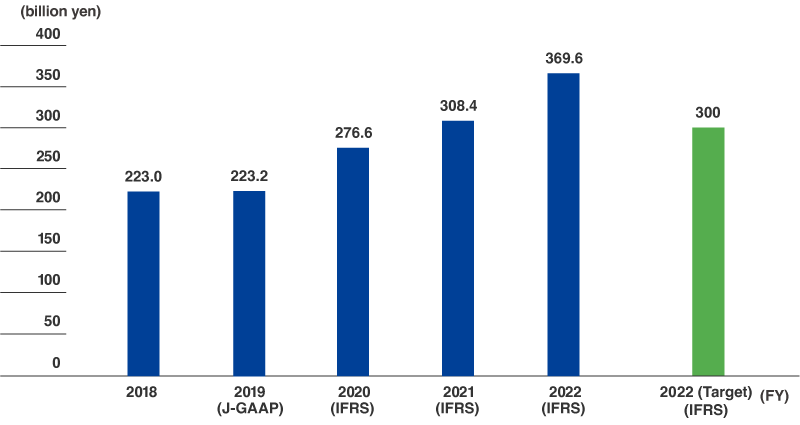
Note: FY2020-22 performance and FY2022 target are revenue based on International Financial Reporting Standards (IFRS).
Under the Medium-Term Management Program, Project AP-G 2025, launched in fiscal 2023, the Life Innovation business has been combined with the Green Innovation business to create the Sustainability Innovation (SI) business, with the aim of further business expansion.
Life Innovation Product Topics
Toray Launches LIVMOA™ 4500AS Disposable Personal Protective Clothing Conforming with Japanese Standard for Chemical Spray Resistance
 LIVMOA™ 4500AS
LIVMOA™ 4500AS
Toray Industries, Inc. has developed LIVMOA™ 4500AS disposable personal protective clothing. The new offering conforms with the JIS T 8115 Type 4 standard for the chemical spray resistance of chemical protective clothing. It also offers excellent dust protection and breathability and outstanding water resistance from the addition of seam tape.
Toray developed the fabric for this product in 2021. It employs a highly durable SMS (spunbond + dense, water-resistant meltblown + spunbond) nonwoven, antistatic fabric. This fabric protects against dust and can withstand a water pressure of 1,000mm H2O (where the water pressure resistance at seams is less than 1,000 mm H2O1), which is hard to achieve with regular SMS fabric.
Toray believes that LIVMOA™ 4500AS is the world's first clothing to be Type 4-compliant while delivering an air permeability of around 7 cc/cm² per second2.
This new product can provide protection in a variety of tasks in which water resistance is vital. They include controlling dioxin levels at waste incineration facilities and performing major regular factory repairs. They also encompass work at chemical plants, maintenance, working in dirty areas, or removing asbestos.
Since debuting the LIVMOA™ series in 2017, Toray has broadened the lineup to cater to diverse applications, including dust protection, infection control, and clean rooms. It will keep developing offerings that combine comfort and functionality for various needs.
- 1 Seams are resistant to under 1,000mm H20 water pressure.
- 2 Based on Toray research
Toray Develops Antiviral Particles3 that Deactivate Viruses Almost Immediately
Conventional disinfection with antiseptic solutions and other chemicals is effective and fast-acting on viral infections. The downside, however, is short volatilization, necessitating regular disinfections. While non-volatile metal-based antivirals offer generally lasting protection, the issue is that many of them take at least an hour to deactivate 99.9% of viruses.
Toray responded to that situation by developing antiviral particles that deactivate 99.9% or more of SARS-CoV-2 virus (the cause of COVID-19) strains in just 15 seconds and 99.99% or more of the strains within 5 minutes. The company achieved this by adding virus adsorption and oxidative degradation capabilities to cerium oxide particles through proprietary synthesis and surface treatment techniques. Toray drew on functional particle design, synthesis, and surface control technologies that it has developed over the years. The new particles deactivate viruses around 100 times faster than conventional metal-based antiviral agents. They are thus among the world’s quickest deactivation delivery vehicles.
Another benefit is that the particles do not volatilize and are lasting protection. That is because they do not use the virus deactivation principle of slow releases with drugs, metal ions, or other active ingredients. The particles also offer excellent safety and resist discoloration and corrosion.
Prospective media for the particles include building materials, paints, and packaging materials. They could thus be deployed in diverse products, including in public spaces in which numerous people might gather, necessitating measures to safeguard from virus infection. Other targets could be public transportation facilities, restaurants, medical and eldercare facilities, interior walls and railings at schools, and regular appliances and food packaging.
Antiviral particles could coat or be kneaded into diverse items. These could include non-woven fabrics for Toray’s masks and medical gowns, air filters, car seats, and other products that could benefit from these particles to prevent droplet and contact infections. Toray will gradually roll out test samples of the particles to customers.
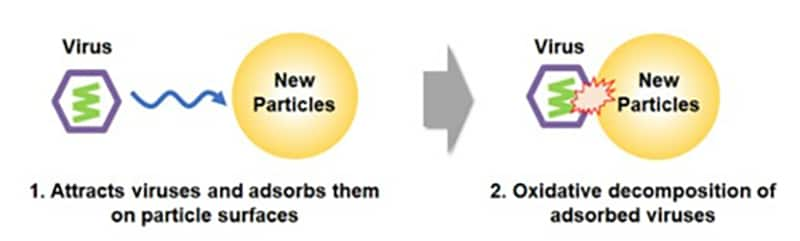
- 3 The creation of these particles stemmed partly from a joint research effort with Professor Satoshi Taharaguchi of the School of Veterinary Medicine at Azabu University under the New Energy and Industrial Technology Development Organization’s Feasibility Study Program on Materials and Biotechnology.
Toray HotBalloon™ Ablation Catheter Qualifies for Insurance to Treat Persistent Atrial Fibrillation
 SATAKE・HotBalloon™ ablation catheter
SATAKE・HotBalloon™ ablation catheter
Toray has secured insurance coverage for the SATAKE・HotBalloon™ ablation catheter currently sold by Toray Medical Co., Ltd. to treat drug refractory recurrent symptomatic persistent atrial fibrillation in Japan. The company is authorized domestically to manufacture and sell this product.
Atrial fibrillation is an irregular and often very rapid heart rhythm from abnormal electrical signal in the atria. There are four main types of such fibrillation; paroxysmal (terminates within 7 days), persistent (sustained beyond 7 days), long-standing persistent (sustained for at least 12 months) and permanent atrial fibrillation.
Around 70,000 ablations for atrial fibrillation are performed in Japan each year. An estimated 20% of those procedures are for the persistent type.
Toray secured marketing approval in 2015 for the SATAKE・HotBalloon™ ablation catheter to treat drug refractory recurrent symptomatic paroxysmal atrial fibrillation. There has been a growing need in recent years to also use this product for persistent atrial fibrillation.
The company accordingly obtained marketing approval in October 2021 to add HotBalloon™ to indications for treating drug refractory recurrent symptomatic persistent atrial fibrillation, with insurance coverage beginning in May 2022. Toray expects this additional indication can be a new treatment option for persistent atrial fibrillation patients.
Toray Files Manufacturing and Marketing Approval for In Vitro Diagnostic Test of Changing Apolipoprotein A2 Isoform Concentrations in Blood of Pancreatic Cancer Patients
On June 27, 2022, Toray filed a manufacturing and marketing approval with the Ministry of Health, Labour and Welfare for an in vitro diagnostic test to measure apolipoprotein A2 (APOA2) isoform concentrations in blood.
APOA2 is a major component of high-density lipoprotein (HDL), and comprises a 77-amino acid chain. There is a carboxyl (C)-terminal amino acid sequence of alanine (A), threonine (T), and glutamine (Q). The APOA2-ATQ full-length protein and APOA2-AT C-terminal degraded isoform coexist in blood.
Professor Kazufumi Honda of the Graduate School of Medicine of Nippon Medical School discovered that the quantitative ratios of APOA2-AT and APOA2-ATQ change in the blood of pancreatic cancer patients (see four references below). He previously led the Department of Biomarkers for Early Detection of Cancer at the National Cancer Center Japan.
Toray has furthered on his research in developing a reagent to measure APOA2 isoform as an in vitro diagnostic test. The company is collaborating with the Nippon Medical School, leveraging research findings from the National Cancer Center and Japan Agency for Medical Research and Development.
This reagent measures both APOA2-AT and APOA2-ATQ levels in the blood of individuals with suspected pancreatic cancer. The reagent uses a proprietary Toray antibody in a sandwich enzyme-linked immunosorbent assay.
Click here for the main initiatives for CSR Guideline 7, “Contributing Solutions to Social Issues through Business Activities” in CSR Roadmap 2022.
